Research
A reversible tuning and a persistent (irreversible) modification of physical properties by external stimuli are one of the main challenges in materials science. Recently, current-induced insulator-metal transition, electrochromism, photo-induced magnetization, magnetic-field induced structural phase transtion, electrochemical control of magnetization etc. have been reported. Especially, photo-control over the physical properties is important from the viewpoint of the practical application as well as the basic science. The photo-tunable compounds can be used future memory devices, optical switches and so on.
- O. Sato, J. Tao, Y. Zhang, Control of Magnetic Properties through External Stimuli. Angew. Chem. Int. Ed. 46, 2152-2187 (2007).
- O. Sato, Nature Chem. 2, 10-11 (2010).
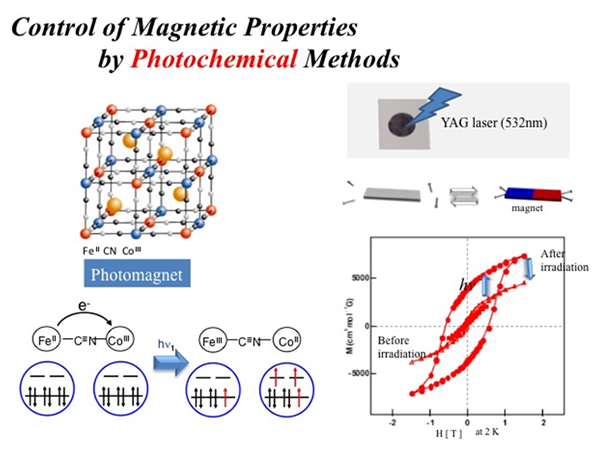
Photo-Induced Magnetization in FeCo Prussian blue
We discovered that FeCo Prussian blue exhibits photo-induced magnetization effects. Photo-illumination effects were studied by exciting the metal-to-metal charge transfer (MMCT) band from FeII-LS to CoIII-LS at around 550nm. When the sample was illuminated at 5 K in the SQUID, the increase of the magnetization value was observed. This suggests that the charge transfer from Fe to Co was induced by illumination. That is, following reaction proceeds, FeII(t2g6eg0)-CN-CoIII-LS(t2g6eg0) -> FeIII(t2g5eg0)-CN-CoII-HS(t2g5eg2). Field-cooled magnetization (FCM) curve displayed no abrupt breaks before illumination. However, when they are illuminated by the 500-750nm light at 5 K, the abrupt breaks at Tc = 26K was observed. The field dependence of the magnetization at 2K showed that the magnetization value was significantly increased after illumination. The magnetization (M) vs. H curve measured at 2K before illumination did not show any hysteresis loops, since it is a paramagnetic compound. In contrast, the plot of M vs. H after illumination did exhibit hysteresis loops. These results demonstrate that the paramagnetic material was converted to a magnetic material by illumination.
- O. Sato, T. Iyoda, A. Fujishima, and K. Hashimoto, Science 272, 704-705 (1996).
- O. Sato, Y. Einaga, T. Iyoda, A. Fujishima, and K. Hashimoto, Inorg. Chem. 38, 4405-4412(1999).
- N. Shimamoto, S. Ohkoshi, O. Sato, and K. Hashimoto, Inorg. Chem. 41, 678-684 (2002).
- O. Sato, Acc. Chem. Res. 36, 692-700 (2003).
- O. Sato, T. Kawakami, M. Kimura, S. Hishiya, S. Kubo, and Y. Einaga, J. Am. Chem. Soc. 126, 13176-13178 (2004).
- T. Liu, D.-P. Dong, S. Kanegawa, S. Kang, O. Sato, Y. Shiota, K. Yoshizawa, S. Hayami, S. Wu, C. He, C.-Y. Duan, Angew. Chem. Int. Ed. 51, 4367-4370, (2012).
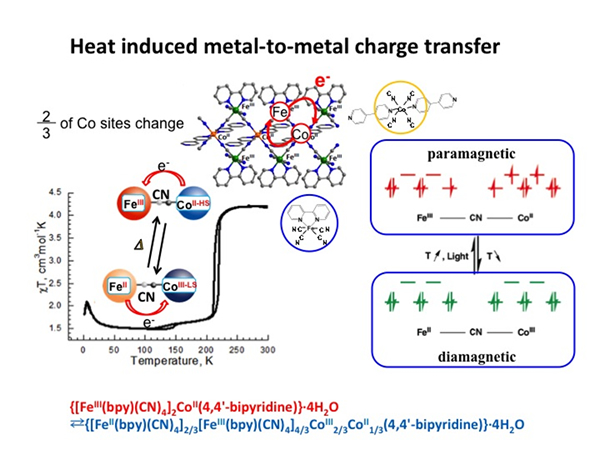
Photo-Tunable Quantum Magnets
In order to prepare a phototunable Single Chain Magnet (SCM) designing of 1-D magnets with negative anisotropy, strong intrachain magnetic interaction, and well-isolated structure between 1-D chains was required. Moreover, 1-D compounds were expected to exhibit charge transfer or spin crossover, which can be achieved by designing the appropriate energy level and ligand field of metal ions. On the basis of this strategy, we synthesized an FeCo complex, [Fe(bpy)(CN)4]2Co(4,4′-bipyridine), with neutral bimetallic layers. The FeCo complex exhibited thermally induced intramolecular charge transfer between FeIII–CN–CoII–HS and FeII–CN–CoIII–LS. Furthermore, on light irradiation at 5 K, charge transfer from FeII to CoIII was induced and a metastable FeIIICoII state was trapped. Variable-temperature ac susceptibility measurements revealed a significant frequency dependence of both in-phase (χ′) and out-of-phase components (χ′′), as observed in other SCMs. The pre-exponential factor and the relaxation energy barrier were τ0 = 1.4 × 10−9 s and Δτ/kB = 29 K, respectively. A semicircular Cole–Cole diagram gave an α value of 0.34. In-phase ac susceptibility showed a frequency-independent peak at 3.8 K, which is due to weak interchain antiferromagnetic interactions. Hence, the magnetic properties after irradiation should be an antiferromagnetic phase of SCM.
- T. Liu, Y.-J. Zhang, S. Kanegawa, O. Sato, J. Am. Chem. Soc., 132, 8250-8251 (2010).
- D.-P. Dong, T. Liu, S. Kanegawa, S. Kang, O. Sato, C. He, C. Y. Duan, Angew. Chem. Int. Ed. 51, 5119-5123 (2012).
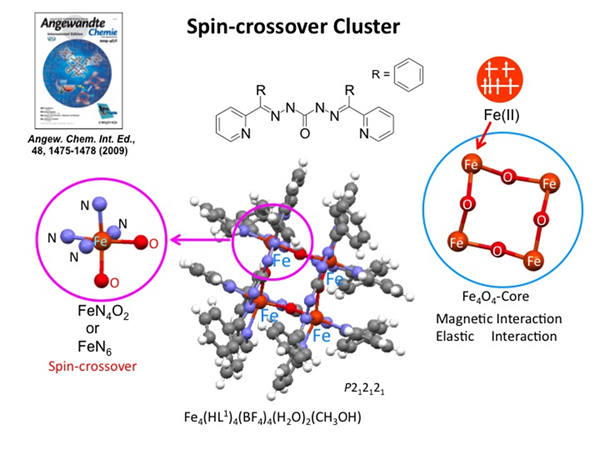
Light-Induced Excited Spin State Trapping in Fe(II) Spin Crossover Clusters
We have synthesized a spin-crossover grid, [Fe4(HL1)4]•(BF4)4•(H2O)2•CH3OH, showing synergy between spin crossover and the magnetic interaction. The magnetic property exhibits a two-step decrease in the magnetization value. On cooling from room temperature, spin crossover occurs from the FeII–HS4 to FeII–HS2FeII–LS2 state at around 170 K. These two FeII–HS have a cis-type arrangement in the cluster. The formation of two HS and LS states results from the presence of intracluster anticooperative interactions. On further cooling, the χMT value approaches 0 cm3mol−1K with decreasing temperature because of the presence of antiferromagnetic interactions between two FeII–HS sites. When this complex is irradiated at 5 K by 532-nm light to excite the metal to ligand charge transfer (MLCT) band, only a small increase was observed in magnetization. This is due to the operation of the antiferromagnetic interaction in the cluster. The ground state with an FeII–HS2FeII–LS2 structure before irradiation has S = 0 because of the antiferromagnetic interaction between two FeII–HS ions. Furthermore, the ground state after irradiation also has S = 0 because of the antiferromagnetic interaction between neighboring FeII ions in the FeII–HS4 state. Hence, only a small increase in magnetization was observed at low temperatures although two HS species were generated after irradiation. This demonstrated that the grid is a spin-crossover cluster in which the magnetic interaction operates between FeII–HS sites, which is distinguishable from typical spin crossover complexes.
- D. Y. Wu, O. Sato, Y. Einaga, C.-Y. Duan Angew. Chem. Int. Ed. 48, 1475-1478 (2009).
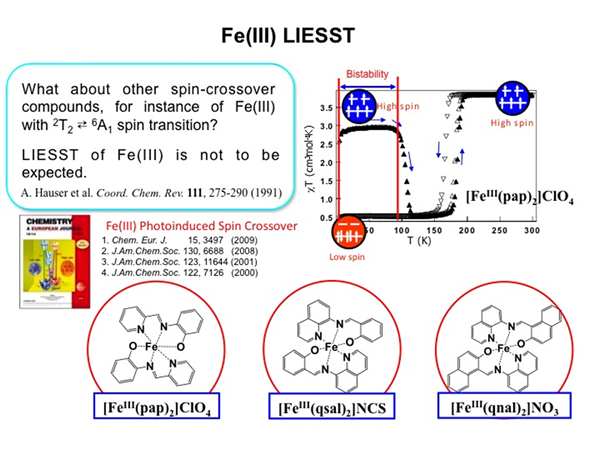
Light-Induced Excited Spin State Trapping in Fe(III) Spin Crossover Complexes
LIESST effects were only observed for FeII complexes in solid state, and the observation of the LIESST effect in FeIII and CoII molecular solids was unexpected. On the other hand, we have recently succeeded in observing the LIESSTeffects in FeIII complexes for the first time.The FeIII complex, [FeIII(pap)2]•PF4•MeOH, exhibits spin crossover at around 288K. When the sample was illuminated at 5 K, the increase of the magnetization value was observed. This means that the transition from low-spin to high-spin, FeIII-LS(t2g5eg0) -> FeIII-HS(t2g3eg2), was induced by illumination. Furthermore, it was found that the metastable state relaxed to the original state, when the sample was heated to above ca. 60K. This is a first report on the FeIII LIESST effects. In analogy to a previous work, it is thought that illuminating the FeIII complex with visible light induces the LMCT, followed by relaxation to the high-spin state.
- S. Hayami, Z.-Z. Gu, M. Shiro, Y. Einaga, A. Fujishima, and O. Sato, J. Am. Chem. Soc. 122 7126-7127 (2000).
- S. Hayami, Z.-Z. Gu, Y. Yoshiki, A. Fujishima, and O. Sato, J. Am. Chem. Soc. 123, 11644-11650 (2001).
- K. Takahashi, H.-B. Cui, Y. Okano, H. Kobayashi, H. Mori, H. Tajima, Y. Einaga, O. Sato J. Am. Chem. Soc. 130, 6688–6689 (2008).
Photoinduced Valence Tautomerism in Co Complexes
The study of the optical and magnetic properties in valence tautomeric compounds has recently attracted great attention. Recently, we reported that the Co valence tautomeric compound does exhibit long-lived charge transfer at low temperature as in the case of the LIESST effects. The photo-effect was studied by exciting the 3,5-dbcat to CoIII-LS charge transfer band of the Co complex, [CoIII-LS(3,5-dbsq)(3,5-dbcat)(phen)], at 5K. When the sample was illuminated at 5 K, the magnetization value was increased. This means that the transition from [CoIII-LS(3,5-dbsq)(3,5-dbcat)(phen)] to [CoII-HS(3,5-dbsq)2(phen)] was induced by illumination. It can be expressed by[CoIII-LS(3,5-dbsq)(3,5-dbcat)(phen)] -> [CoII-HS(3,5-dbsq)2(phen)]. The change in the magnetization persisted for periods of at least an hour after the illumination was stopped. Furthermore, when the sample was heated after illumination, the metastable state was relaxed back to the original state at around 50K. Similar photoinduced valence tautomerism with long lifetime was also observed in other several Co valence tautomeric compounds. Furthermore, it was found that the reverse valence tautomeric reaction could be induced by exciting the charge transfer band from CoII-HS to 3,5-dbsq.
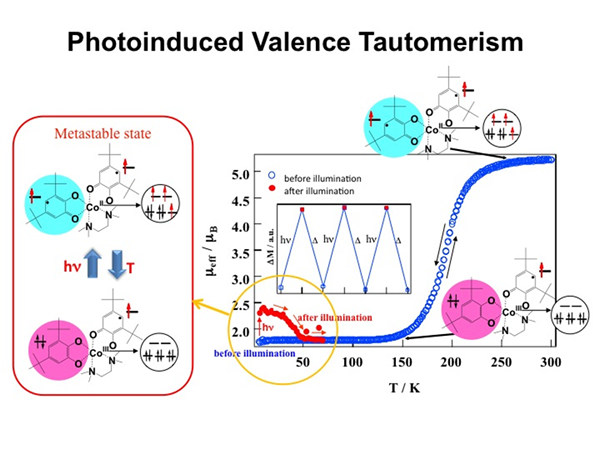
- O. Sato, S. Hayami, Z.-Z. Gu, K. Seki, R. Nakajima, and A. Fujishima, Chem. Lett. 874-875 (2001).
- O. Sato, S. Hayami, Z.-Z. Gu, K. Takahashi, R. Nakajima, and A. Fujishima, Chem. Phys. Lett. 355, 169 (2002) .
- J. Tao, H. Maruyama, O.Sato, J. Am. Chem. Soc. 128, 1790-1791 (2006).
- O. Sato, A.L. Cui, R. Matsuda, J. Tao, S. Hayami, Acc. Chem. Res. 40, 361-369 (2007).
Photo-Tunable Photonic Band Gap Crystals
Composite materials comprised of nematic liquid crystals (LCs) and SiO2 inverse opal films were fabricated. Their optical properties were quite different from those of inverse opal films without the LCs. The optical properties could be controlled by changing the refractive indices of the LCs, which vary with orientation, phase and temperature. In particular, the optical properties were drastically changed by thermal or photo-induced isothermal phase transitions of the LCs. This means that the photonic band structure could be controlled, and tunable photonic crystals have been achieved, based on the inverse opal structure. The mechanism of this change was investigated by the evaluation of the effective refractive indices. As a result, it was found that the change in optical properties was derived from the orientation of the LC molecules in the voids in the inverse opal film. Furthermore, once the mechanism was understood, it was also possible to control the position of the reflection peak by changing the alignment of the LCs. Such materials have the possibility for practical use in optical devices and fundamental research systems.
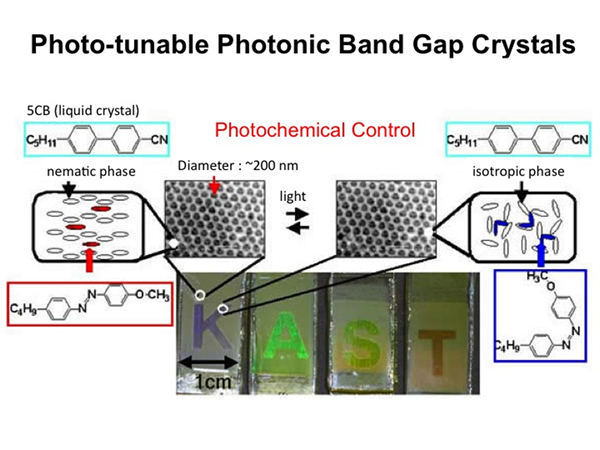
- Gu, Z.-Z., Fujishima, A. & Sato, O. J. Am. Chem. Soc. 122, 12387-12388 (2000).
- Gu, Z.-Z. Meng, Q. -B., Hayami, S., Iyoda, T., Fujishima, A. & Sato, O. J. Am. Chem. Soc. 122, 10730-10731 (2000).
- Kubo, S. Gu, Z. -Z., Takahashi, K., Ohko, Y., Sato, O. & Fujishima, A. J. Am. Chem. Soc. 124, 10950-10951 (2002).
- Kubo, S., Gu, Z.-Z. Takahashi, K., Fujishima, A., Segawa, H. & Sato, O. J. Am. Chem. Soc. 126, 8314-8319 (2004).
- O. Sato, S. Kubo, Z.-Z Gu. Acc. Chem. Res. 42, 1-10 (2009).
Orbital Angular Momentum
Control of magnetic properties can be achieved by changing spin multiplicity. The change in spin multiplicity is realized by inducing charge transfer and spin crossover. However, magnetic susceptibility is also affected by orbital angular momentum. Hence, the bistability of magnetization can be achieved by changing the contribution of the orbital angular momentum term. The quenching of orbital angular momentum depends on the ligand field and hence on the structure around metal ions. Hence, in order to realize thermally induced change in orbital angular momentum, we synthesized a Co complex, [CoII(NO3)2(L')] (L' = 2,6-di(pyrazol-1-yl)pyrazine), incorporating NO3 ligands. The octahedral HS CoII complex has typically unquenched orbital angular momentum. Furthermore, NO3 is a flexible ligand; therefore, we anticipated that the temperature-dependent structural change would be induced in the complex. Indeed, this complex exhibited structural phase transition at approximately 235 K. As a result, the change in magnetization accompanying the hysteresis loop was clearly observed. Because spin multiplicity does not change, the switching in magnetization can be achieved from the change in orbital contribution, which is induced by the modification of the structure around CoII–HS ions.
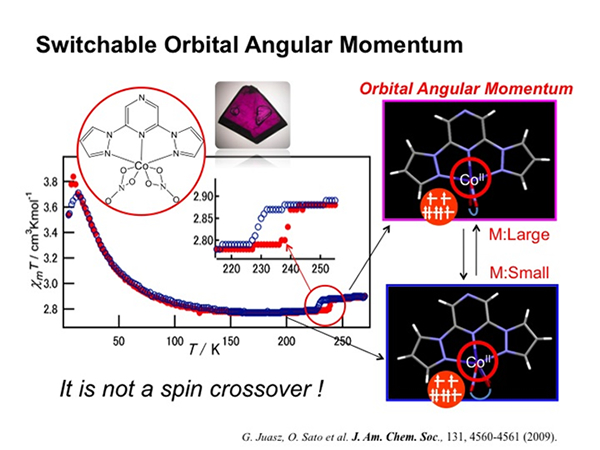
- Juhasz, G., Matsuda, R., Kanegawa, S., Inoue, K., Sato, O. and Yoshizawa, K. J. Am. Chem. Soc. 131, 4560-4561 (2009).







